Espresso notes
These are a loose collection of notes about espresso brewing.
Water treatment
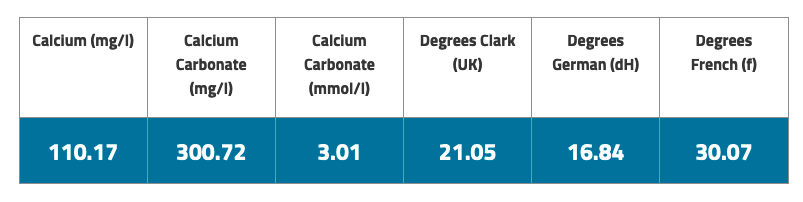
Water hardness is a function of two main components: calcium and magnesium. Hardness is typically measured in mg/L of equivalent calcium carbonate, which is found by adding together the concentration of calcium and magnesium ions with multipliers of 2.5 and 4.1, respectively. The formula is here. Typically, magnesium is only a small component, the dominant component to water hardness is calcium. The total is often called GH (general hardness).
Here is a scale of water hardness (from here.
| Water hardness (in mg/L of CaCO3) | |
| Soft | up to 100 |
| 100-150 | Slightly hard |
| 150-200 | Moderately hard |
| 200-300 | Hard |
| 300+ | Very hard |
Here are some data points of comparison.
- My area of Bath has water hardness of 300 mg/L (source
- Back in my hometown, Ottawa, Canada, the hardness is 30 mg/L (source)
Here are two clear sources: